n a groundbreaking development, the U.S. Food and Drug Administration (FDA) has granted approval for two pioneering treatments, Casgevy and Lyfgenia, as the first cell-based gene therapies for sickle cell disease (SCD) in patients aged 12 and older. Notably, Casgevy represents a significant leap in innovation, being the first FDA-approved treatment to incorporate CRISPR/Cas9, a revolutionary genome editing technology.
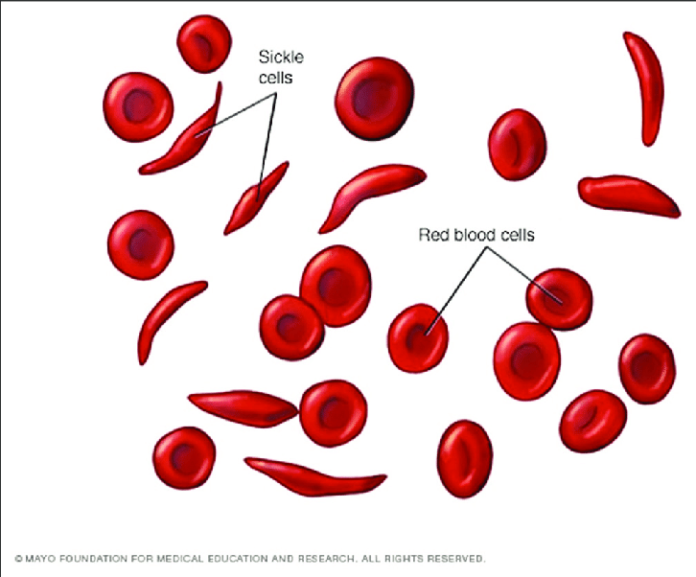
Sickle cell disease is a hereditary blood disorder affecting around 7·74 million individuals globally with a higher prevalence among those of African descent. The condition stems from a mutation in hemoglobin, the protein responsible for oxygen transport in red blood cells. This mutation results in the distinctive crescent or “sickle” shape of red blood cells, causing vaso-occlusive events (VOEs) or vaso-occlusive crises (VOCs), leading to severe pain and organ damage.
Director of the FDA’s Center for Biologics Evaluation and Research, Nicole Verdun, M.D., expressed excitement about advancing treatments for sickle cell disease. “Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited,” she said.
Casgevy, one of the approved therapies, employs CRISPR/Cas9 technology to modify patients’ hematopoietic stem cells. This genome editing technique precisely targets and alters DNA, enhancing the production of fetal hemoglobin (HbF) in the modified cells. Elevated levels of HbF prevent the sickling of red blood cells in individuals with sickle cell disease.
Lyfgenia, the second approved therapy, utilizes a lentiviral vector for genetic modification, resulting in the production of HbAT87Q—a gene-therapy derived hemoglobin that mimics normal adult hemoglobin A. Red blood cells containing HbAT87Q have a reduced risk of sickling and obstructing blood flow.
Both Casgevy and Lyfgenia involve a one-time, single-dose infusion of modified blood stem cells, collected from the patient before undergoing myeloablative conditioning, a process that facilitates the engraftment of modified cells within the bone marrow.
Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, emphasized the rigorous evaluation process, stating, “These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health.”